All individual trials at Nationwide Children's Hospital
Trials reported
6 out of 12

Percent reported
50.0%

US Govt could have imposed fines of at least
$72,676,458
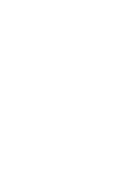
Fines claimed by US Govt
$0
Showing 1 to 33 of 33 entries
| Status | Sponsor | Trial ID | Title | Completion date | Days overdue |
|---|---|---|---|---|---|
| reported-late | Nationwide Children's Hospital | NCT03734822 | Comparative Analysis of CO2 Monitoring Methods in Patients With CF Undergoing General Anesthesia [pACT] | 2017-12-20 | 39 |
| reported-late | Nationwide Children's Hospital | NCT03054922 | Impact of Intraoperative Fluid Management on Electrolyte and Acid-base Variables | 2018-09-30 | 163 |
| reported-late | Nationwide Children's Hospital | NCT03650842 | Changes in Cerebral Oxygenation During Laparoscopic Pyloromyotomy | 2018-10-08 | 42 |
| overdue | Nationwide Children's Hospital | NCT01667120 | The Use of Ketorolac in Surgical Neonates [pACT] | 2018-12-31 | 1501 |
| overdue | Nationwide Children's Hospital | NCT02793011 | Does Dexamethasone Reduce Postoperative Pain in Pediatric Tonsillectomy Patients? [pACT] | 2019-06-27 | 1322 |
| overdue | Nationwide Children's Hospital | NCT03379974 | Exercise Versus DDAVP in Patients With Mild Hemophilia A - is One Non-inferior to the Other and do They Work Additively in Improving Hemostasis? | 2019-08-30 | 1258 |
| overdue | Nationwide Children's Hospital | NCT01270724 | A Phase II Clinical Trial of Induction Chemotherapy Regimen Gemcitabine, Paclitaxel and Oxaliplatin (GemPOx) Followed by a Single Cycle of High Dose Chemotherapy (HDC) and Autologous Hematopoietic Stem Cell Rescue (AuHSCR) for Patients With Recurrent or Progressive Intracranial Germ Cell Tumors [pACT] | 2019-10-23 | 1204 |
| reported-late | Nationwide Children's Hospital | NCT03746951 | Comparative Evaluation of Lumbar Plexus and Suprainguinal Fascia Iliaca Compartment Blocks for Pain Management After Orthopedic Surgical Procedures Involving Hip and Femur in Pediatrics. | 2020-01-31 | 374 |
| overdue | Nationwide Children's Hospital | NCT03230032 | RCT of Feeding Intervention With Pacifier Activated Device and Mother's Voice in Infants at High-risk for Cerebral Palsy. | 2021-07-31 | 558 |
| reported-late | Nationwide Children's Hospital | NCT03982342 | Preliminary Percutaneous Intervention Versus Observational Trial of Arterial Ductus in Low-weight Infants (PIVOTAL) | 2021-08-31 | 111 |
| reported | Nationwide Children's Hospital | NCT04757805 | Assessment of the Accuracy of the Manual Palpation of Surface Landmarks Versus Ultrasound for Identification of the Correct Intervertebral Space for Spinal Anesthesia in Children Less Than 1 Year of Age | 2022-03-21 | |
| overdue | Nationwide Children's Hospital | NCT03276065 | Randomized Controlled Trial of Laser Hair Depilation in Adolescents With Pilonidal Disease | 2022-12-31 | 40 |
| ongoing | Nationwide Children's Hospital | NCT03729999 | Ultrasound to Verify Lung-isolation During Single-lung Ventilation | 2023-11-27 | |
| ongoing | Nationwide Children's Hospital | NCT03769844 | GM-CSF for Reversal of immunopAralysis in pediatriC sEpsis-induced MODS (GRACE) | 2023-12-05 | |
| ongoing | Nationwide Children's Hospital | NCT05975684 | A Randomized Controlled Pilot Trial of Baclofen for Children With Rumination Syndrome | 2023-12-31 | |
| ongoing | Nationwide Children's Hospital | NCT04211675 | A Phase I/II Safety Lead in Study of Ex-Vivo Expanded Allogeneic Universal Donor TGFβi NK Cell Infusions in Combination With Irinotecan, Temozolomide, and Dinutuximab in Patients With Relapsed or Refractory Neuroblastoma: The Allo - STING Trial | 2023-12-31 | |
| ongoing | Nationwide Children's Hospital | NCT03520751 | Phase I/IIa Trial Evaluating scAAV1.tMCK.NTF3 for Treatment of Charcot-Marie-Tooth Neuropathy Type 1A (CMT1A) | 2024-03-01 | |
| ongoing | Nationwide Children's Hospital | NCT04722029 | Open-Label Pilot Study of Haploidentical Donor Adenovirus Specific T Lymphocytes (ADV-VSTS) for the Treatment of Refractory Adenovirus Infection and/or Disease in Hospitalized Patients | 2024-10-01 | |
| ongoing | Nationwide Children's Hospital | NCT02875314 | HeadStart4: Newly Diagnosed Children (<10 y/o) With Medulloblastoma and Other CNS Embryonal Tumors Clinical and Molecular Risk-Tailored Intensive and Compressed Induction Chemotherapy Followed by Consolidation With Randomization to Either Single Cycle or to Three Tandem Cycles of Marrow-Ablative Chemotherapy With Autologous Hematopoietic Progenitor Cell Rescue [pACT] | 2024-12-31 | |
| ongoing | Nationwide Children's Hospital | NCT05233397 | Phase 2 Study of Systemic IL-6 Receptor Antagonist ACTEMRA® (Tocilizumab) for the Treatment of Progressive/Recurrent Pediatric Adamantinomatous Craniopharyngioma | 2024-12-31 | |
| ongoing | Nationwide Children's Hospital | NCT03431090 | HAPLEUK17, Haploidentical Hematopoietic Cell Transplantation for Children With Hematologic Malignancies and Myelodysplasia | 2024-12-31 | |
| ongoing | Nationwide Children's Hospital | NCT02378428 | A Phase II Study of <131>I-Metaiodobenzyguanidine (<131>I-MIBG) Therapy for Patients With MIBG Avid Tumors [pACT] | 2025-03-31 | |
| ongoing | Nationwide Children's Hospital | NCT05286788 | Phase 2 Study of the MEK Inhibitor MEKTOVI® (Binimetinib) for the Treatment of Pediatric Adamantinomatous Craniopharyngioma | 2025-04-10 | |
| ongoing | Nationwide Children's Hospital | NCT04467671 | Prospective, Open-labeled, Single-arm Clinical Trial to Evaluate the Safety and Efficacy of the Second-generation Tissue Engineered Vascular Graft as Vascular Conduits for Extracardiac Total Cavopulmonary Connection. | 2025-08-31 | |
| ongoing | Nationwide Children's Hospital | NCT05135975 | Phase 2 Study of Cabozantinib as a Maintenance Agent to Prevent Progression or Recurrence in High-Risk Pediatric Solid Tumors | 2025-12-31 | |
| ongoing | Nationwide Children's Hospital | NCT05547165 | Percutaneous Intervention Versus Observational Trial of Arterial Ductus in Low Weight Infants | 2026-02-28 | |
| ongoing | Nationwide Children's Hospital | NCT05634369 | A Multi-Institution Study of TGFβ Imprinted, Ex Vivo Expanded Universal Donor NK Cell Infusions as Adoptive Immunotherapy in Combination With Gemcitabine and Docetaxel in Patients With Relapsed or Refractory Pediatric Bone and Soft Tissue | 2026-12-31 | |
| ongoing | Nationwide Children's Hospital | NCT05503134 | Killer Cells Against Relapsed/Refractory Myeloid Acute Leukemia (KARMA): A Clinical Trial Evaluating the Safety and Efficacy of Expanded, Universal Donor Natural Killer Cells for Treatment of Young Adults With Relapsed/Refractory Acute Myeloid Leukemia | 2027-02-28 | |
| ongoing | Nationwide Children's Hospital | NCT05267821 | Targeted Reversal of Inflammation in Pediatric Sepsis-induced MODS (TRIPS) | 2027-05-15 | |
| ongoing | Nationwide Children's Hospital | NCT05266001 | GM-CSF for Reversal of Immunoparalysis in Pediatric Sepsis-induced MODS | 2027-05-31 | |
| ongoing | Nationwide Children's Hospital | NCT05843253 | PhaseII Study of Ribociclib and Everolimus Following Radiotherapy in Pediatric and Young Adult Patients Newly Diagnosed With HGG Including DIPG, Which Harbor Alterations of the Cell Cycle and/or PI3K/mTOR Pathways | 2028-01-15 | |
| ongoing | Nationwide Children's Hospital | NCT05096481 | Phase 2 Trial of a Novel Peptide Vaccine (PEP-CMV) Targeting CMV Antigen for Newly Diagnosed Pediatric High-grade Glioma and Diffuse Intrinsic Pontine Glioma and Recurrent Medulloblastoma | 2028-04-15 | |
| ongoing | Nationwide Children's Hospital | NCT06082947 | αβT Cell/CD19+ B Cell Depletion for Alternative Donor Allogeneic Hematopoietic Cell Transplantation (HSCT) for Children and Young Adults With Hematologic Malignancies | 2028-12-01 |
