All individual trials at New York Medical College
Trials reported
2 out of 12

Percent reported
16.7%

US Govt could have imposed fines of at least
$108,922,135
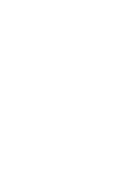
Fines claimed by US Govt
$0
Showing 1 to 27 of 27 entries
| Status | Sponsor | Trial ID | Title | Completion date | Days overdue |
|---|---|---|---|---|---|
| reported-late | New York Medical College | NCT01636388 | A Multicenter Pilot Study of Reduced Intensity Allogeneic Stem Cell Transplantation Followed by Adoptive Cellular Immunotherapy With Donor Derived Latent Membrane Protein (LMP) Specific-CTLs in Patients With Epstein-Barr Virus (EBV)Positive Refractory or Recurrent Hodgkin Lymphoma [pACT] | 2017-06-30 | 1839 |
| reported-late | New York Medical College | NCT01049854 | CD34+Stem Cell Selection for Patients Receiving Partially Matched Family or Matched Unrelated Adult Donor Allogeneic Stem Cell Transplantations for Malignant and Non-Malignant Disease [pACT] | 2017-11-30 | 1687 |
| overdue | New York Medical College | NCT01834911 | Effect of Tetrabenazine on Stroop Interference in Huntington Disease [pACT] | 2018-01-01 | 1865 |
| overdue | New York Medical College | NCT03719729 | A Phase II Study of Rifaximin (Xifaxan) for Patients With Sickle Cell Disease (SCD) | 2020-02-22 | 1082 |
| overdue | New York Medical College | NCT06103656 | Double-Blind, Placebo-Controlled Phase 2 Study to Assess Safety and Efficacy of Chinese Herbal Therapy and Multiple Food Allergen Oral Immunotherapy [pACT] | 2020-07-02 | 952 |
| overdue | New York Medical College | NCT02851472 | Prevention of Transfusion Related Acute Gut Injury (TRAGI) in Extremely Low Gestational Age Neonates (ELGAN) Neonates Using iNO | 2021-02-05 | 734 |
| overdue | New York Medical College | NCT04041843 | A Pilot Study of an Oral Anticoagulant "Apixaban" for theTreatment of Venous Thromboembolism in Children and Adolescents | 2021-05-31 | 619 |
| overdue | New York Medical College | NCT02098512 | A Multicenter Pilot Study of Reduced Intensity Conditioning and Allogeneic Stem Cell Transplantation Followed by Targeted Immunotherapy in Children, Adolescents and Young Adults With Poor Risk CD30+ Hodgkin Lymphoma (HL) [pACT] | 2021-12-01 | 435 |
| overdue | New York Medical College | NCT03805581 | A Phase II Study to Determine the Safety of Defibrotide in Sickle Cell Disease-Related Acute Chest Syndrome | 2022-07-01 | 223 |
| overdue | New York Medical College | NCT03613987 | Randomized Control Trial: Synchronized Non-Invasive Positive Pressure Ventilation (sNIPPV) With Neurally-Assisted Ventilatory Assist (NAVA) Versus NIPPV in Extremely Low Birth Weight Infants | 2022-07-26 | 198 |
| overdue | New York Medical College | NCT04071600 | Intranasal Neuropeptide Y in Clinical Trial in Level Two Trauma Patients for PTSD and Acute Stress Disorder (ASD) | 2022-10-31 | 101 |
| overdue | New York Medical College | NCT02558972 | Northera Improves Postural Tachycardia Syndrome (POTS) and Postural [pACT] | 2022-12-31 | 40 |
| ongoing | New York Medical College | NCT02117297 | Allogeneic Stem Cell Transplantation Following by Targeted Immune Therapy) (Gemtuzumab Ozogamicin) in Average Risk Acute Myelogenous Leukemia and Myelodysplastic Syndrome (AML/MDS) [pACT] | 2023-03-30 | |
| ongoing | New York Medical College | NCT02221310 | A Pilot Study of Gemtuzumab Ozogamicin in Combination With Busulfan and Cyclophosphamide (Immunochemotherapy) and Allogeneic Stem Cell Transplantation in Patients With High Risk Acute Myelogenous Leukemia and Myelodysplastic Syndrome [pACT] | 2023-04-04 | |
| ongoing | New York Medical College | NCT04478617 | PROJECT PREVENT: Metronidazole Antibiotic Per Vagina Before Hysterectomy: Is Additional Antibiotic Prophylaxis Beneficial? | 2023-05-31 | |
| ongoing | New York Medical College | NCT04777422 | A Pilot Study to Determine the Safety of Defibrotide in Children With High Risk Kawasaki Disease | 2023-10-24 | |
| ongoing | New York Medical College | NCT01461837 | Familial Haploidentical T-Cell Depleted Transplantation in High-Risk Sickle Cell Disease (IND 14359) [pACT] | 2023-12-31 | |
| ongoing | New York Medical College | NCT04197596 | A Pilot Study in the Treatment of Refractory BK Infections With Related Donor BK Specific Cytotoxic T-cells (CTLs) in Children, Adolescents and Young Adult Recipients | 2023-12-31 | |
| ongoing | New York Medical College | NCT04491370 | Safety and Tolerability of Myeloablative Conditioning and Autologous Stem Cell Transplantation Followed by Polatuzumab Vedotin (PV) Immunoconjugate Therapy in Patients With B-cell Non-Hodgkin and Hodgkin Lymphoma | 2024-08-15 | |
| ongoing | New York Medical College | NCT03266640 | A Pilot Study in the Treatment of Refractory Cytomegalovirus (CMV) Infections With Related Donor CMV Specific Cytotoxic T-cells (CTLs) in Children, Adolescents and Young Adult Recipients | 2024-08-31 | |
| ongoing | New York Medical College | NCT03266653 | A Pilot Study in the Treatment of Refractory Epstein-Barr Virus (EBV) Infection With Related Donor EBV Cytotoxic T-Lymphocytes in Children, Adolescents and Young Adult Recipients | 2024-08-31 | |
| ongoing | New York Medical College | NCT03266627 | A Pilot Study in the Treatment of Refractory Adenovirus (ADV) Infection With Related Donor ADV Cytotoxic T-Lymphocytes (ADV-CTLs)in Children, Adolescents and Young Adult Recipients | 2024-08-31 | |
| ongoing | New York Medical College | NCT04896606 | A Pilot Study of SARS-CoV-2 Specific Cytotoxic T Lymphocytes (SARS-CoV-2-CTLs) for Treatment of Mild to Moderate Coronavirus Disease 2019 (COVID-19) | 2024-12-31 | |
| ongoing | New York Medical College | NCT02675959 | Safety and Efficacy of Prophylactic Defibrotide in Children, Adolescents, and Young Adults With Sickle Cell Disease or Beta Thalassemia Following MAC and Haploidentical Stem Cell Transplantation Utilizing CD34 Enrichment and T-Cell (CD3) Addback | 2024-12-31 | |
| ongoing | New York Medical College | NCT05987124 | A Phase II Intrapatient Open-Label Dose Escalation Trial of Defibrotide in Hematopoietic Cell Transplantation (HCT) Recipients With Sinusoidal Obstructive Syndrome (SOS) Post-HCT Associated With Either Renal and/or Pulmonary Dysfunction With Either Refractory or Progressive Disease Following Defibrotide Therapy | 2026-08-01 | |
| ongoing | New York Medical College | NCT05564598 | A Phase II Open-Label Randomized Study of Anti-Viral Antibiotic Therapy With and Without Familial (Maternal) Cytomegalovirus (CMV) Cytotoxic T Lymphocytes (CTLs) in Neonates With Moderate/Severe Maternal Acquired CMV Infection | 2027-10-31 | |
| ongoing | New York Medical College | NCT05253495 | Reducing the Burden of Oncologic Chemoradiotherapy And Radiation Exposure From Diagnostic Imaging by Utilizing Targeted Immunotherapy in Children, Adolescents and Young Adults With Lymphoma | 2027-12-31 |
